Hypoglycemia in people with type 1 diabetes can be easily treated with a ready-to-use glucagon pen

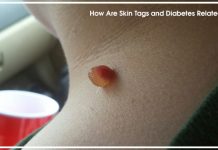
Very low blood sugar level in a type 1 diabetes is referred to as hypoglycemia. Severe hypoglycemia requires immediate medical attention as it can be very dangerous, if not attended to immediately.
The conventional treatment of hypoglycemia requires mixing of glucagon powder with a mixing solution, and then giving injection to the patient. These injections are an integral part of the conventional glucagon kits. This procedure can be easily performed at home but requires a series of steps.
The latest Dasiglucagon pen is developed by the Danish firm Zealand Pharma. It further simplifies the process of using conventional glucagon kits. The glucagon pen can be very beneficial for people during emergency situations owing to its ready-to-use feature.
The research was conducted on 58 people with type-1 diabetes by injecting insulin intravenously. After the insulin induced hypoglycemia, the glucagon pen was tested on these participants. The researchers closely monitored the glucose levels after using glucagon pens. The research was done to compare the conventional glucagon kits with the new ready-to-use glucagon pens. The researchers also measured whether the pen was effective or not, how quickly it acted and whether there were any unbearable side effects of the pen.
The research found that the glucagon pens acted as quickly as the conventional glucagon kits, but the pens had a long-lasting and greater effects of raising the blood glucose levels. The side effects were also similar to those induced by the conventional glucagon kits. However, nausea was a frequently observed side effect of both the glucagon pen and the conventional kits.
It is expected that this simpler version of glucagon treatment would be commercially available soon. The study was recently published in the Diabetes Care Journal in the December 2017 issue.