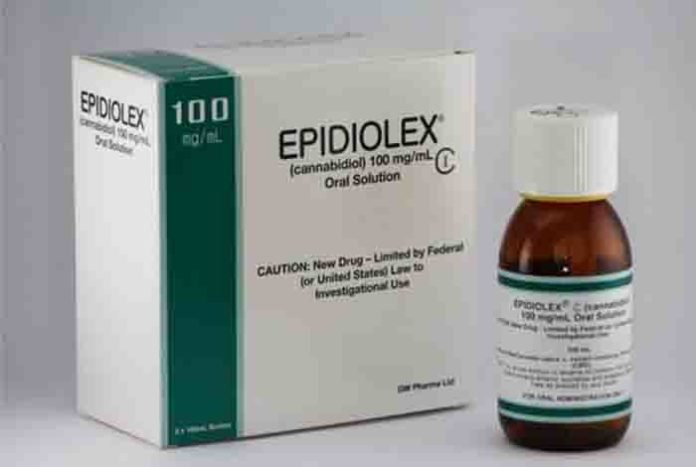
First prescription cannabidiol medicine has been voted by Federal Advisors for recommendation to get approval for treating epilepsy. Even the US. FDA advisory committee has voted in unison for the approval of the marijuana-derived drug, known as Epidiolex.
Cannabidiol or CBD is derived from cannabis plant. It will be approved only for treating seizures that result from Dravet syndrome and Lennox-Gastaut syndrome in patients aged 2 years and above.
Epidiolex is a silver lining in the dark clouds for people, who live with unmanageable seizures and epilepsy. Unquestionably, it is a hope for people, who face challenging and uncontrollable seizures and live under the threat of injury and death.
CBD is being sold as a sleep enhancer and cure for cancer. It is also portrayed as a pain as well as anxiety reliever. However, most claims are not backed by medical studies. In fact, the U.S. FDA has warned companies that make them.But Epidiolex is different.
A British organization, GW Pharmaceuticals has conducted necessary tests for the consideration of the U.S. FDA drug approval.
Its specially and carefully prepared oral form of medicine is required to be made under strict standards, known as good manufacturing practices; so that buyers can rely on what is being sold to them. Also Dr. Orrin Devinsky, director at NYU epilepsy center says that as a physician, it is utterly important to know what is being prescribed to the patients and what is given to them for one month will be the same as what will be given to them for four or six months later.
Devinsky adds that it is important to know that cannabidiol, used in this medicine, is derived from cannabis and purified up to 99%.
You won’t be able to find it in a dispensary or drug store in the U.S. You also won’t get it from smoking marijuana. It is a pharmaceutical product obtained from marijuana, but not at all similar to what is being sold in the U.S presently.
The FDA will approve the drug and doctors may prescribe it as any other FDA-approved drug if they find it appropriate for some condition. But, the prescribed use of this product in extremely limited.
It is not known how CBD works but studies claim that it works. The FDA has said that the studies give enough evidence that CBD is effective in the treatment of seizures related to conditions like Lennox-Gastaut syndrome and Dravet syndrome. The risks of CBD treatment are acceptable if we consider the fact that Lennox Gastaut(LG) and Dravet syndrome (DS) are life-threatening and debilitating disorders.
The panelists listened to aggrieved parents, including Lisa Smith, whose daughter has DS and suffered countless seizures. She tried 17 medications and their combinations by age of 14, but nothing worked.FDA Committee
She was told by the doctors that since there was nothing left, they would prescribe CBD provided they wouldn’t be arrested.
DS is a rare and severe form of epilepsy, which results from genetic mutation. Those suffering with DS experience multiple seizures, causing brain damage. LG is also rare, leading to seizures, and results in cognitive impairment.
Devinsky talks about some children he takes care of, who experience many seizures a day, are on wheelchairs and medications, and either sleeping or going through seizures. Some of these children are not on wheelchair now and they are out in the school, running, playing and enjoying life with their families. It is nothing short of a magical transformation.
Doctors also want to use CBD in treating autism, autoimmune, inflammatory disorders and anxiety.
It is not the same as medical marijuana as it does not have THC – a substance that causes a euphoric high. Medical marijuana has been approved in some 29 U.S. states. Studies claim that it is helpful in treating anxiety and relieves symptoms of multiple sclerosis (MS) and glaucoma.
Many marijuana based products have been approved by the FDA including Marinol which is used in treatment of nausea and appetite loss as in AIDS.
There are many cases of epilepsy being reported in the U.S. The numbers touched 2.3 million in 2010.