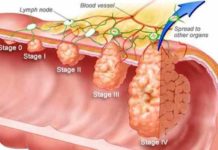
Study suggests delays in colon cancer treatment doesn’t increase death risk

A recent study reported that delays in the treatment of colon cancer do not increase death risk.
A clinical research collected the data from 900 people, who were diagnosed with stage 1 to stage 3 colon cancer. The analysis, conducted by Canadian scientists, examined the duration of the treatment adopted by the patients.
The researchers reported that some of the people started the treatment within a month of their diagnosis, some started after 30 to 59 days, some 60 to 89 days, some after 90 to 119 days, while a few took more than 4 months for initiating the treatment after the diagnosis. It was calculated that the average time between the diagnosis and starting the treatment was 38 days.
As per the reports of the analysis, there was no connection between the delay in the treatment and the survival rate of the people. The study was published in December issue of a journal, Diseases of the Colon and Rectum.
Study author, Dr. Kerollos Wanis, Western University, Ontario said that their research may prove to be helpful while deciding the appropriate time for surgical treatment of cancer. He also added, “We also hope that our study will assuage provider and patient anxiety regarding surgical treatment delay, particularly when additional preoperative workup and optimization is required.”
Source: philly.com