Abbott, a global leader in diabetes care, has got the much-awaited approval from the U.S. Food and Drug Administration (FDA) for marketing the revolutionary new continuous glucose monitoring (CGM) system, called FreeStyle Libre.
The system is intended to be used by people having insulin-dependent diabetes. It is being viewed as a system that will replace the traditional finger-prick blood glucose monitoring system. It is free from daily finger stick calibrations and real-time readings and can be used for insulin dosing. Its main aim is to provide a system that is approachable, accessible and affordable for 30 million diabetics living in the U.S.
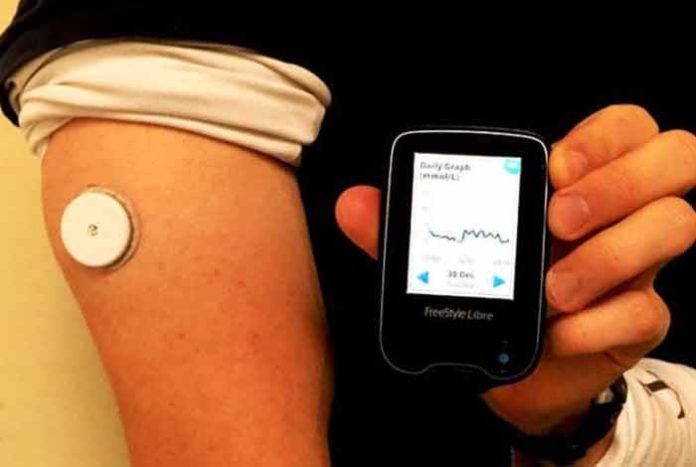
The system consists of only two pieces – a sensor and a reader – to measure the blood glucose level. A thin, flexible filament is inserted painlessly just under the skin to measure glucose levels in interstitial fluid. It gives an estimated value of A1C in the blood. The sensor can be scanned from within 1.5 inches using the reader.
The device can be worn on the back of the upper arm. It is water resistant and hence can be used while bathing, exercising and swimming. The device automatically records glucose level every 15 minutes to provide more insightful data. There is no interaction of the patient with the sensor. No separate receiver, transmitter or recorder are to be worn by the patient. An added advantage of this device is that it does not get affected by acetaminophen. It can store eight hours of recent glucose data. Presently, it does not have smartphone connectivity, which the company plans to have soon.
The device has been in use in European countries since 2014. Globally, people have shown positive response to the device and is being used by more than 4 million people in nearly 41 countries.