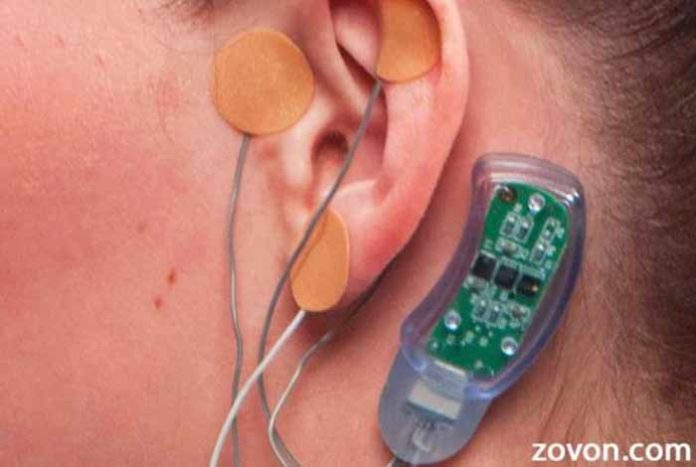
For reducing the symptoms of opioid withdrawal, a novel device has been approved by the U.S. Food and Drug Administration (FDA). This is an electrical nerve stimulator.
Innovative Health Solutions Inc. designed the device, which is placed behind the patient’s ear and left for five days, i.e., the duration of acute withdrawal phase.
The device is known as NSS-2 Bridge and it has been finally approved after a trial on over 73 patients, who were going through the phase of opioid withdrawal. The trial reported that there was a reduction of nearly 31% in the symptoms of opioid withdrawal.
The symptoms of withdrawal that are reduced with the device include sweating, tremors, stomach upset, joint pain and anxiety.
Source: Fox News.com