FDA approval for new Shingles vaccine, Shingrix

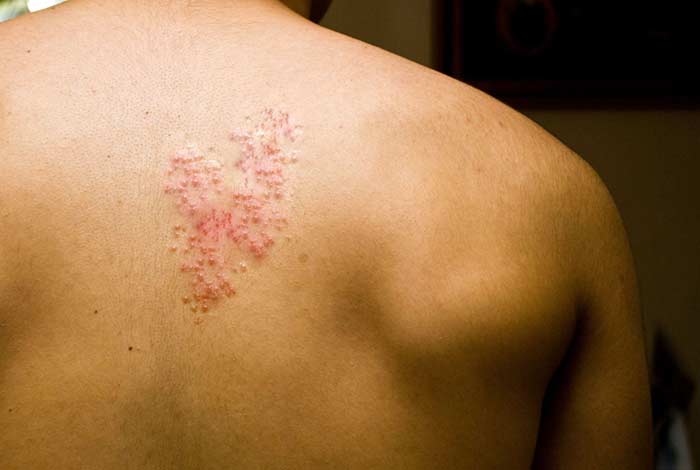
The U.S. Food and Drug Administration (FDA) approved Shingrix as a new vaccine for shingles as a more effective option than the previous Zostavax vaccine, made by Merck and licensed since 2006. The U.S. Advisory Committee on Immunization Practices (ACIP) support Shingrix over Zostavax as a vaccine to prevent shingles – a painful skin condition characterized by rashes and caused by chickenpox virus in older people.
Previously, Shingles vaccine was recommended for age sixty and above. But, according to Washington Post’s report, this new vaccine could be effective in people aged fifty and above.
According to a study conducted by GlsxoSmithKline – the manufacturer of Shingrix, the earlier vaccine Zostavax protected just fifty percent patients with one shot, while, the new Shingrix protected ninety percent patients with two shots. The former is slightly cheaper than the latter and essentially covered under many medical insurance plans.
The ACIP recommended that those, who had already been vaccinated with Zostavax, should be revaccinated with Shingrix. According to Post’s report, 20 million people belong to this group those will be re-vaccinated by Shingrix.
A medical officer from the U.S. Centers for Disease Control and Prevention (CDC), Kathleen Dooling said, “This represents a major advance for people who want to be protected against the disease and its complications.”
Experts, who supervised the study, stated that the research was conducted through clinical experiments that include large number of participants belonging to different age groups. The future of Shingrix was decided by a 15-member panel of the ACIP, where Shingrix won over Zostavax in a close contest that resulted 8-7 voting, as suggested by Washington Post. Cynthis Pellegrini, a member of the committee, who had voted against the vaccine proposal told CNN, “It’s not so much a matter of not preferring (Shingrix); It’s a matter of not preferring this vaccine at this particular moment in time.”
Shingrix is just a step away to be approved by the CDC Director. Once done, it will be published through new guideline policy by early next year.